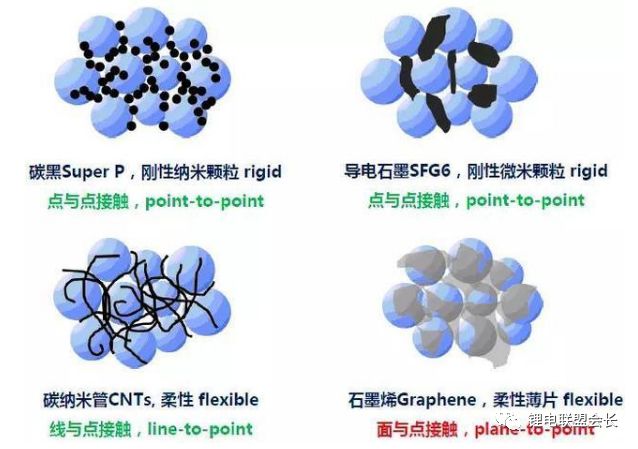
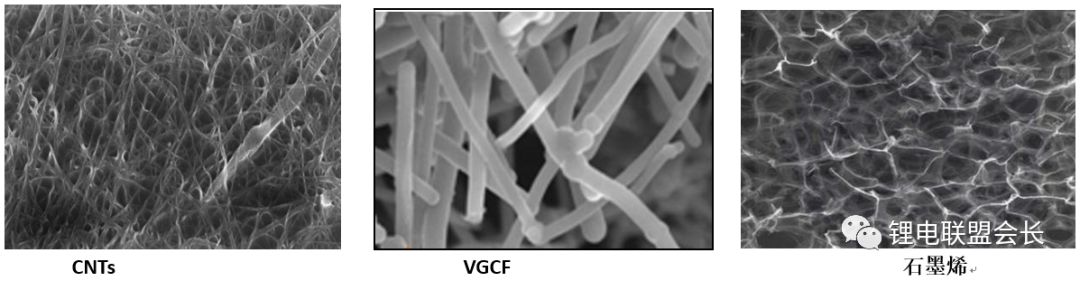
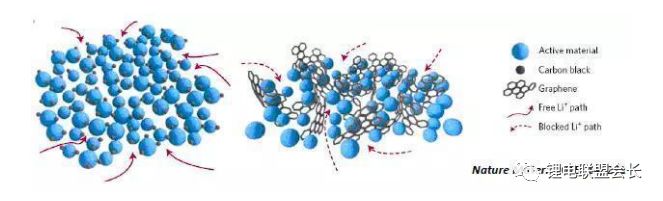
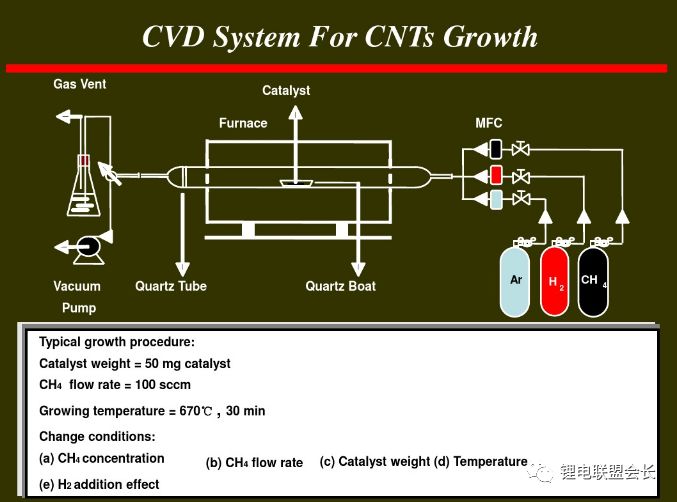
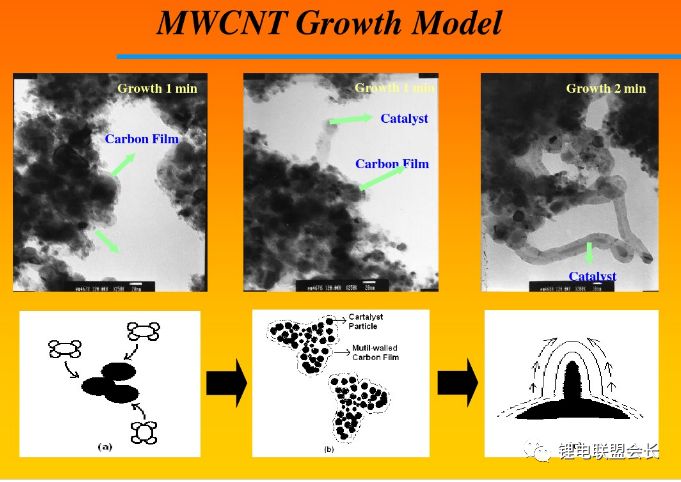
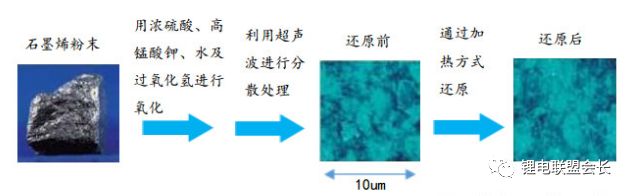
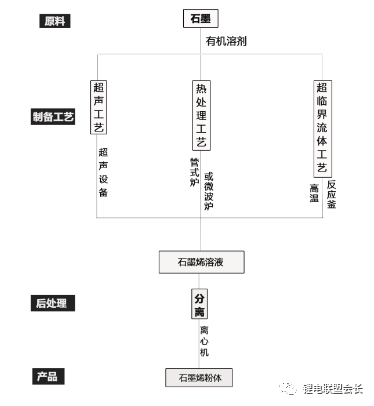
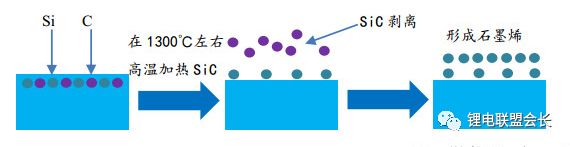
The conductive agent, which is one of the key non-main materials of lithium ion batteries, plays a very important role in the entire lithium ion pole piece. Its most basic function is to conduct electricity. In the manufacture of a pole piece, a certain amount of conductive material is usually added to make it active. Collecting microcurrents between substances, between the active material and the current collector, to reduce the contact resistance of the electrode to accelerate the movement rate of the electrons, and at the same time, it can effectively increase the migration rate of lithium ions in the electrode material, thereby increasing the The charge and discharge efficiency of the electrode, this article starts with the commonly used conductive agent, to the majority of lithium colleagues to popularize the basic knowledge and application of conductive agents. 1. Classification and Overview of Conductive Agents From the above table, it can be seen that the specific surface area of ​​the conductive agent is gradually increasing, the particles are also getting smaller and smaller, and the oil absorption value is getting larger and larger. For the current lithium-ion battery market, the positive electrode conductive agent is used The most common ones are CNTs, CNTs and SPs, CNTs and graphenes, etc., all of which are attempts to form a three-dimensional conductive network. The negative electrode conductive agent is mainly SP, and the following is the conduction mechanism of each material. Conduct the analysis. It can be seen from the figure that both SP and conductive graphite SFG6 are rigid nanoparticles that form point contacts between the material and the particles. In the low-end lithium-ion market, SP is still widely used as a positive electrode conductive agent. It has better ion and electronic conductivity, and has a larger specific surface area, so it is conducive to the adsorption of electrolytes to improve the ionic conductivity, and its primary particles are agglomerated to form a branched chain structure and can form a chain-type conductive structure with active materials. Helps increase the electronic conductivity of the material. However, with the increase of energy density of the power battery, the SP alone cannot satisfy the performance of the power battery, and it is necessary to develop a better conductive agent. Therefore, CNTs, VGCF, graphene, etc. have been developed. For CNTs, used as a conductive agent in combination with SP, a continuous conductive network can be formed in the electrode active material; after the addition, the pole piece has high toughness, and can improve the peeling caused by the change in the volume of the material during charge and discharge, and increase the cycle life. Can greatly improve the electrolyte penetration ability in the electrode material, but due to its larger specific surface area, there will be a certain degree of difficulty in the homogenization of the slurry. Currently available on the market are 5% or so slurry, directly Used when homogenizing; Graphene, which has recently become very hot, can also act as a conductive agent for CNTs. However, for the time being, the advantages of lithium are not yet realized, and its relatively difficult homogenization process and high price And the fact that the surface has a large number of functional groups to bring about the subsequent conductivity problems, etc., will bring certain problems to its application, and it still takes a long way to go. 2. Method for synthesizing conductive agent Conductive carbon black synthesis method Synthesis of CNTs Graphene synthesis method: Micro mechanical peeling In 2004, Geim et al. for the first time successfully stripped and observed monolayer graphene from highly oriented pyrolytic graphite using a micro mechanical lift-off method. Geim's research group successfully used this method to prepare quasi-two-dimensional graphene and observed its morphology, revealing the existence of two-dimensional crystal structure of graphene. Micro mechanical stripping method can produce high quality graphene, but it has the disadvantages of low yield and high cost. It does not meet the requirements of industrialization and large-scale production. At present, it can only be prepared as a small-scale laboratory. Chemical Vapor Deposition Chemical Vapor Deposition (CVD) has made new breakthroughs in the large-scale production of graphene for the first time. The CVD method refers to a chemical reaction of a reactant substance under a gaseous condition to produce a solid state material that is deposited on a surface of a heated solid substrate to obtain a solid material. Kong et al. of the Massachusetts Institute of Technology, Hong et al. of Sungkyunkwan University in Korea, and Chen et al. of Purdue University used graphene to prepare CVD. They use a tubular nickel-based simple deposition furnace that passes carbon-containing gas, such as hydrocarbons, which decomposes at high temperatures into carbon atoms that deposit on the surface of nickel to form graphene, passing slightly. The chemical etching etched the graphene film and the nickel sheet to obtain a graphene film. This kind of film can reach 1.1×106S/m when the light transmittance is 80%, which is a potential alternative to the current transparent conductive film. High-quality large-area graphene can be prepared by CVD, but the ideal substrate material, monocrystalline nickel, is too expensive, which may be an important factor affecting the industrialization of graphene production. The CVD method can meet the requirements of large-scale preparation of high-quality graphene, but the cost is high and the process is complicated. Oxidation-reduction method Oxidation-reduction method is cheap and easy to implement, and it is the best method for preparing graphene. It can also prepare stable graphene suspension and solve the problem that graphene is not easy to disperse. Oxidation-reduction method refers to the reaction of natural graphite with strong acid and strong oxidizing substance to generate graphite oxide (GO). After ultrasonic dispersion, it is prepared into graphene oxide (monolayer graphite oxide), and a reducing agent is added to remove the oxygen-containing surface of the graphite oxide. The groups, such as carboxyl, epoxy and hydroxyl groups, give graphene. After the oxidation-reduction method was proposed, it became the simplest method for preparing graphene in the laboratory with its simple and easy process, and it was widely favored by graphene researchers. Ruoff et al. found that graphene can be obtained by adding a chemical substance such as dimethylhydrazine, hydroquinone, sodium borohydride (NaBH4), liquid hydrazine, or the like to remove an oxygen-containing group of graphene oxide. The oxidation-reduction method can prepare a stable graphene suspension, and solves the problem that graphene is difficult to disperse in a solvent. The disadvantage of the oxidation-reduction method is that macro-preparation can easily lead to waste liquid pollution and there are certain defects in the prepared graphene, for example, five-membered ring, seven-membered ring or other topological defects or structural defects of -OH groups, which will The loss of electrical properties of the graphene portion results in the limitation of the application of the graphene. Solvent stripping The principle of the solvent stripping method is to disperse a small amount of graphite in a solvent to form a low-concentration dispersion, and the van der Waals force between the graphite layers is destroyed by the action of an ultrasonic wave. At this time, the solvent can be inserted between the graphite layers and peeled off to prepare a layer. Graphene. This method does not destroy the structure of graphene like the oxidation-reduction method, and high-quality graphene can be prepared. The graphene yield is highest (about 8%) in the nitrogen methylpyrrolidone and the conductivity is 6500 S/m. It has been found that highly-oriented pyrolytic graphite, thermally-expanded graphite, and microcrystalline artificial graphite are suitable for the preparation of graphene by solvent stripping. The solvent stripping method can produce high quality graphene. The entire liquid phase stripping process does not introduce any defects on the surface of the graphene, which provides a broad application prospect for its application in the fields of microelectronics, multifunctional composite materials and the like. The disadvantage is that the yield is very low. Solvothermal method The solvothermal method refers to the preparation of materials in a closed reaction vessel (autoclave) using an organic solvent as the reaction medium and heating the reaction system to a critical temperature (or close to the critical temperature) to generate a high pressure in the reaction system itself. An effective method. The solvothermal method solves the problem of large-scale preparation of graphene, and at the same time brings about a negative effect of low electrical conductivity. In order to solve the deficiencies caused by this, the researchers combined solvothermal method and redox method to produce high-quality graphene. Dai et al. found that the graphene thin film prepared by reduction of graphene oxide under solvothermal conditions is smaller than graphene prepared under conventional conditions. The characteristics of solvothermal method for preparing high-quality graphene under high temperature and high pressure closed system are more and more concerned by scientists. The combination of solvothermal method and other preparation methods will become another bright spot for the preparation of graphene. 3. The future development trend of conductive agents With the increase of energy density of power batteries, the requirements for conductive agents are also rising. Based on the years of R&D experience of Lao Li, the development trend of conductive agents is mainly in the following aspects; 1) Environmental protection With the development of technology, the positive electrode is also gradually using water as a solvent, which requires the conductive agent not only to be oleophilic, but also to be hydrophilic and easy to disperse; 2) Decrease in content The content of active substances is getting higher and higher, and the content of non-active substances is getting lower and lower. This requires that the amount of conductive agent must be small, and at the same time, it can also play a very good conductive effect; 3) The use of three-dimensional conductive network composite conductive agent, whether it is point contact, line contact, surface contact, etc., are to build a three-dimensional conductive network, conducive to lithium ion shuttle; 4) Simple synthesis and low price Summary: This article mainly introduces the more important component of the lithium-ion battery auxiliary material--conductive agent, which is introduced in detail from the aspects of classification, conduction mechanism, production methods, and future development trends. I believe the majority of lithium-ion colleagues are Conductive agents have a preliminary understanding, but in the actual use process, it is necessary to consider how to choose a suitable conductive agent and its content in combination with the types of actual materials, the method of homogenization, and the properties of the battery to be satisfied. It requires a combination of long-term practical experience and theory. It is believed that in the future, more and better conductive agents will appear in people's vision. Hdmi Fiber,Fiber Optic Hdmi,Fiber Hdmi Cable,Fiber To Hdmi Dongguan Tuojun Electronic Technology Co., Ltd , https://www.fibercablessupplier.com